
Biosafety
The Institutional Biosafety Committee (IBC) was established under the provisions of the NIH Guidelines for Research Involving Recombinant DNA Molecules (NIH Guidelines). The role of the IBC is to establish and implement policies that provide for the safe conduct of recombinant DNA research and to ensure compliance with the NIH Guidelines. In addition, the IBC oversees research involving infectious agents and toxic biological molecules. The IBC also reviews research protocols from grant applications submitted to extramural funding agencies to confirm that experiments will be conducted safely and in compliance with the NIH Guidelines. Members of the IBC are appointed by the Senior Vice President for Research and Biotechnology and include persons who are not otherwise affiliated with the University to represent the interests of the surrounding community with respect to health and protection of the environment.
Principal Investigators are responsible for ensuring IBC review of the following:
- Animal experiments requiring IBC approval with any of the above (prior to IACUC approval)
- Biological Toxins (Select Agent toxins below the permissible toxin amount)
- Human and Non-human Primate (NHP): blood, body fluids, tissues and primary or established cell lines
- Pathogens/Microorganisms: Risk Group 1 or higher
- Recombinant or Synthetic DNA work as defined by the NIH Guidelines
Most importantly, the IBC’s objective is to ensure that such activities meet standards of good biological safety practices, emphasizing protection of personnel, the public, and the environment.
All communication should be directed to the IBC office at: ibc@westernu.edu
WesternU IBC utilizes CITI for all compliance related training. Please follow the instructions for registration and completion of IBC required courses though CITI.
IBC related forms can be found in the IRBnet Forms/templates library www.irbnet.org under Western University of Health Sciences IBC – Documents for Researchers. For questions about IBC forms or IRBNet please contact IBC@westernu.edu 909-469-5606.
From your IRBnet page: select Forms/Templates tab, then Select the Library “Western University of Health Sciences IBC Documents for Researchers” from the drop down. 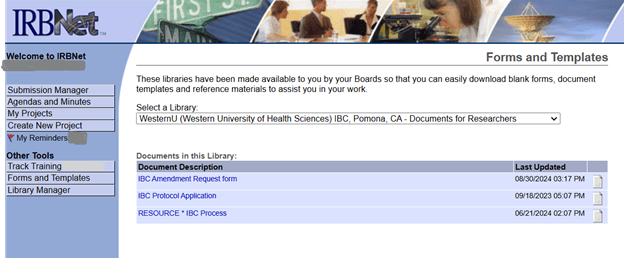
Forms Available:
IBC Application
IBC Amendment Request Form
National Institutes of Health (NIH)
- NIH Requirements for IBC’s
- The NIH Process for Human Gene Transfer Trials- Frequently Asked Questions
Centers for Disease Control (CDC)
Biosafety in Microbiological and Biomedical Laboratories
Federal Select Agent Regulations:
Possession, Use and Transfer of Select Agents and Toxins
Health and Human Services Regulations:
- Interstate Shipment of Etiologic Agents (42 CFR 72)
- Etiologic Agent Import Permit Program (42 CFR 71)
Department of Labor Occupational Safety & Health Administration (OSHA)
- Occupational Exposure to Bloodborne Pathogens (29 CFR 1910.1030)
- Bloodborne Pathogens and Needle Stick Prevention
U.S. Department of Agriculture (USDA-APHIS)
- American Type Culture Collection (ATCC)
- International Air Transport Association (IATA) Guidance on Infectious Substances
- American Biological Safety Association (ABSA) Risk Group Classification
- American Biological Safety Association (ABSA) International
- California Department of Industrial Relations


