COMP (CA): Peterfy Lab
Genetic determinants of common metabolic diseases
Research in the Peterfy Laboratory focuses on common metabolic diseases including obesity, diabetes and dyslipidemia. Our goal is to identify novel genes and mechanisms responsible for metabolic abnormalities and provide the foundation for the development of novel therapeutic approaches. The Peterfy Laboratory is affiliated with the College of Osteopathic Medicine of the Pacific.
The overall goal of the Peterfy Laboratory is to understand mechanisms involved in common metabolic conditions such as obesity, diabetes and dyslipidemia. Our research strategy is to identify genes associated with metabolic abnormalities in mouse models or humans, followed by mechanistic studies using molecular, cellular and organismal approaches. To identify genes of metabolic interest, we have been pursuing two general strategies. The first strategy exploits naturally occurring genetic variation in the mouse to discover genes responsible for metabolic phenotypes. In the second strategy, we identify candidate genes based on human genetic data, followed by characterization in engineered mouse models. Current projects in the Peterfy Laboratory focus on dyslipidemia (i.e., abnormal levels of cholesterol and triglycerides in the circulation), a principal risk factor for coronary artery disease and associated mortality. Our studies aim to identify molecular determinants and mechanisms responsible for dyslipidemia to enable the development of novel therapeutic approaches.
The Role of Lipase Maturation Factor (LMF1) in Plasma Lipid Metabolism
The LMF1 gene was discovered in a naturally occurring mutant mouse strain (cld, combined lipase deficient) exhibiting hyperlipidemia. LMF1 is a chaperone residing within the endoplasmic reticulum (ER), where it is required for the post-translational activation of several lipases involved in plasma lipid metabolism: lipoprotein lipase (LPL), hepatic lipase (HL) and endothelial lipase (EL). In the absence of functional LMF1, as in cld mutant mice, these lipases are inactive and triglyceride clearance is impaired, leading to the accumulation of lipids in the circulation. Similar to the cld mouse model, human LMF1 mutations are associated with lipase deficiency and hypertriglyceridemia.
Our current studies on LMF1 aim to:
- Understand the role of LMF1 in the modulation of lipases and plasma lipid levels through the characterization of tissue-specific knock-out mouse models.
- Identify molecular mechanisms regulating LMF1 function using biochemical and molecular biological techniques.
- Explore lipase-independent roles of LMF1 in ER homeostasis using cell biological approaches.
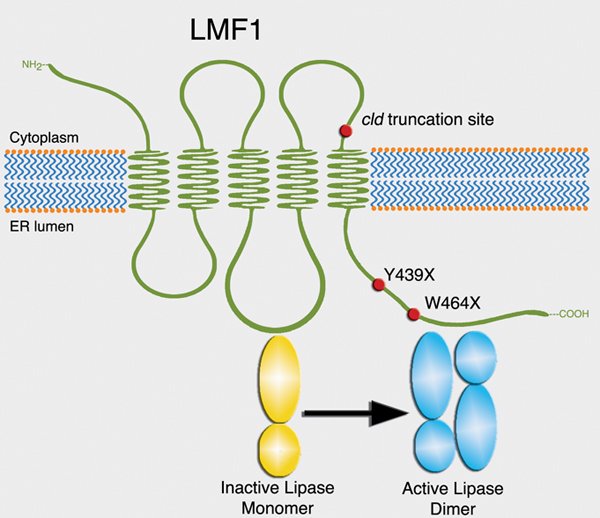 Structure and function of LMF1. Mouse and human mutations associated with hyperlipidemia are indicated with red dots.
Structure and function of LMF1. Mouse and human mutations associated with hyperlipidemia are indicated with red dots.Genetic determinants of plasma lipid variation in human populations
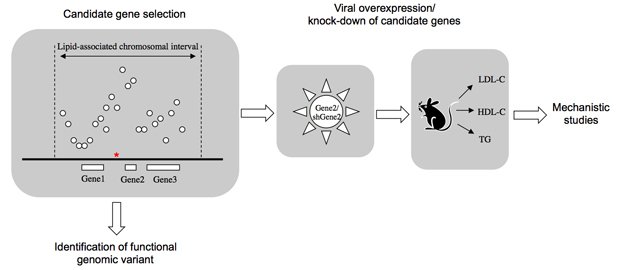
Genome-wide association studies (GWAS) in human populations have uncovered numerous chromosomal regions associated with a variety of metabolic traits, including plasma lipids such as cholesterol and triglycerides. Many of the lipid-associated chromosomal intervals contain genes not previously implicated in lipid metabolism and offer a rich resource for the identification of novel genes and mechanisms affecting this process. Thus, the overall goal of our project is to use human GWAS data as the starting point to discover novel determinants of plasma lipid levels.
Major aims of the project are to:
- Functionally validate candidate genes in lipid-associated genomic intervals using in vivo overexpression and knock-down approaches in mouse models.
- Identify molecular mechanisms by which lipid-associated genes affect plasma lipid levels using in vitro and in vivo approaches.
- Identify causative genomic variants through the functional analysis of lipid-associated haplotypes.

Functional analysis of lipid-associated chromosomal intervals. The functional genomic variant is labeled by red asterisk. )
Funding National Institutes of Health (NHLBI)
See a complete PubMed list of publications by Miklos Peterfy, PhD.
Selected Key Publications
Ehrhardt, N., Cui, J., Dagdeviren, S., Saengnipanthkul, S., Goodridge, H. S., Kim, J. K., Lantier, L., Guo, X., Chen, Y. I., Raffel, L. J., Buchanan, T. A., Hsueh, W. A., Rotter, J. I., Goodarzi, M. O., Peterfy, M. (2019) Adiposity-Independent Effects of Aging on Insulin Sensitivity and Clearance in Mice and Humans. Obesity 27:434-443. Link to publication
Peterfy, M., Bedoya, C., Giacobbe, C., Pagano, C., Gentile, M., Rubba, P., Fortunato, G., Di Taranto, M. D. (2018) Characterization of two novel pathogenic variants at compound heterozygous status in Lipase Maturation Factor 1 (LMF1) gene causing severe hypertriglyceridemia. J. Clin. Lipidol. 12: 1253-1259. Link to publication
Ehrhardt, N., Doche, M. E., Chen, S., Mao, H. Z., Walsh, M. T., Bedoya, C., Guindi, M., Xiong, W., Irudayam, J. I., Iqbal, J., Fuchs, S., French, S. W., Hussain, M. M., Arditi, M., Arumugaswami, V., Peterfy, M. (2017) Hepatic Tm6sf2 overexpression affects cellular ApoB-trafficking, plasma lipid levels, hepatic steatosis and atherosclerosis. Hum. Mol. Gen. 26: 2719-2731. Link to publication
Labadzhyan, A., Cui, J., Peterfy, M., Guo, X., Chen, Y.I., Hsueh, W.A., Rotter, J.I., Goodarzi, M.O. (2016) Insulin clearance is sssociated with hepatic lipase activity and lipid and adiposity traits in Mexican Americans. PLOS One 11: e0166263. Link to publication
Sha H, Sun S, Francisco AB, Ehrhardt N, Xue Z, Liu L, Lawrence P, Mattijssen F, Guber RD, Panhwar MS, Brenna JT, Shi H, Xue B, , Kersten S, Bensadoun A, Peterfy M*, Long Q*, Qi L.* The ER-associated degradation adapter protein Sel1L regulates LPL secretion and lipid metabolism. Cell Metab. 2014;20(3):458-470. *co-senior authors. Link to publication
Mao HZ, Ehrhardt N, Bedoya C, Gomez JA, DeZwaan-McCabe D, Mungrue IN, Kaufman RJ, Rutkowski DT, Peterfy M. Lipase maturation factor 1 (lmf1) is induced by endoplasmic reticulum stress through activating transcription factor 6α (Atf6α) signaling. J Biol Chem. 2014;289(35):24417-24427. .Link to publication
Ehrhardt N, Bedoya C, Peterfy M. Embryonic viability, lipase deficiency, hypertriglyceridemia and neonatal lethality in a novel LMF1-deficient mouse model. Nutr Metab. 2014;11:37-45. Link to publication
Hosseini M, Ehrhardt N, Weissglas-Volkov D, Lai CM, Mao HZ, Liao JL, Nikkola E, Bensadoun A, Taskinen MR, Doolittle MH, Pajukanta P, Peterfy M. Transgenic expression and genetic variation of Lmf1 affect LPL activity in mice and humans. Arterioscler Thromb Vasc Biol. 2012;32(5):1204-1210. Link to publication
Peterfy M. Lipase maturation factor 1: a lipase chaperone involved in lipid metabolism. Biochim Biophys Acta. 2012;1821(5):790-794. Link to publication
Doolittle MH, Ehrhardt N, Peterfy M. Lipase maturation factor 1: structure and role in lipase folding and assembly. Curr Opin Lipidol. 2010;21(3):198-203.Link to publication
Davis RC, Castellani LW, Hosseini M, Ben-Zeev O, Mao HZ, Weinstein MM, Jung DY, Jun JY, Kim JK, Lusis AJ, Peterfy M. Early hepatic insulin resistance precedes the onset of diabetes in obese C57BLKS-db/db mice. Diabetes. 2010;59(7):1616-1625. Link to publication
Cefalu AB, Noto D, Arpi ML, Yin F, Spina R, Hilden H, Barbagallo CM, Carroccio A, Tarugi P, Squatrito S, Vigneri R, Taskinen M-R, Peterfy M, Averna MR. Novel LMF1 nonsense mutation in a patient with severe hypertriglyceridemia. J Clin Endocrin Metab. 2009;94(11):4584-4590. Link to publication
Yin F, Doolittle MH, Peterfy M. A quantitative assay measuring the function of lipase maturation factor 1. J Lipid Res. 2009;50(11):2265-2269. Link to publication
Peterfy M, Ben-Zeev O, Mao HZ, Weissglas-Volkov D, Aouizerat BE, Pullinger CR, Frost PH, Kane JP, Malloy MJ, Reue K, Pajukanta P, Doolittle MH. Mutations in LMF1 cause combined lipase deficiency and severe hypertriglyceridemia. Nat Genet. 2007;39(12):1483-1487. Link to publication